Archives
- 2026-01
- 2025-12
- 2025-11
- 2025-10
- 2025-09
- 2025-03
- 2025-02
- 2025-01
- 2024-12
- 2024-11
- 2024-10
- 2024-09
- 2024-08
- 2024-07
- 2024-06
- 2024-05
- 2024-04
- 2024-03
- 2024-02
- 2024-01
- 2023-12
- 2023-11
- 2023-10
- 2023-09
- 2023-08
- 2023-06
- 2023-05
- 2023-04
- 2023-03
- 2023-02
- 2023-01
- 2022-12
- 2022-11
- 2022-10
- 2022-09
- 2022-08
- 2022-07
- 2022-06
- 2022-05
- 2022-04
- 2022-03
- 2022-02
- 2022-01
- 2021-12
- 2021-11
- 2021-10
- 2021-09
- 2021-08
- 2021-07
- 2021-06
- 2021-05
- 2021-04
- 2021-03
- 2021-02
- 2021-01
- 2020-12
- 2020-11
- 2020-10
- 2020-09
- 2020-08
- 2020-07
- 2020-06
- 2020-05
- 2020-04
- 2020-03
- 2020-02
- 2020-01
- 2019-12
- 2019-11
- 2019-10
- 2019-09
- 2019-08
- 2019-07
- 2019-06
- 2019-05
- 2019-04
- 2018-11
- 2018-10
- 2018-07
-
Based on the close relationship of
2019-04-30
Based on the close relationship of the canine wedge model to human ECGs, individuals with prominent J waves (ERS) or J/ST elevation (BS) are thought to have accentuated ventricular AP notch and the potential to lose their AP dome because of extrinsic factors such as vagal stimulation, sodium channel
-
In February a left neck infiltrating
2019-04-30
In February 2013, a left neck infiltrating purpuric lesion around a previous surgical scar (lymph node biopsy) with mild pain was noted. A subsequent skin biopsy revealed metastatic adenocarcinoma, compatible with ampulla of Vater primary, thereby confirming disease progression with skin metastasis.
-
sphk Varios republicanos espa oles de s lida
2019-04-30
Varios republicanos españoles de sólida posición económica residentes en México habrían recibido esta carta de extorsión, entre ellos personajes como: Fernando Rodríguez Miaja, Máximo Muñoz, Jerónimo Bugeda o Juan Simeón Vidarte, quien se apresuró sphk denunciarlo a las autoridades. Sean ciertas o n
-
Declaration of interests br Abstract Background In times of
2019-04-30
Declaration of interests Abstract Background In times of seawater inundation in coastal deltas, unprotected drinking water sources, such as ponds and shallow tube wells, take on salt water with each inundation. Daily consumption of these saline sources contributes to overall sodium intake. Altho
-
ABT888 Lead failures are classified as
2019-04-30
Lead failures are classified as either structural lead failures or electrical lead failures, and failures of 8F single coil leads are more frequently reported. Structural failures occur with a reported incidence of 14–34% and are evident as cable exteriorization in fluoroscopy studies [6,7]. Electri
-
It is noteworthy that in a recent
2019-04-30
It is noteworthy that in a recent study, Le Scouarnec et al. [199]. estimated the burden of rare coding variation in arrhythmia susceptibility genes among 167 BrS index patients and compared that with 167 individuals ≥65 years old with no history of cardiac arrhythmia. The authors concluded that, ex
-
Two years later he complained of two
2019-04-30
Two years later, he complained of two ICD shocks during a dance party. The possibility of lead fracture or electromagnetic interference was completely excluded after several examinations. Intracardiac electrograms showed that all delivered shocks were inappropriate therapies due to TWOS. The R wave
-
Poor knowledge among caretakers of how to work with
2019-04-30

Poor knowledge among caretakers of how to work with disability-related disorders could also be an issue. For example, poor knowledge of how to correctly position a child with severe cerebral palsy could lead to inadequate feeding; at worst, these efforts can cause aspiration and even death. Social a
-
Ya desde sus investigaciones entre los coras
2019-04-30
Ya desde sus investigaciones entre los coras, Preuss se había percatado de que En otros lugares he amphetamine sulfate argumentado que esta idea preussiana acerca del modo de pensar mágico es la piedra angular de su proyecto antropológico, pues constituye el plexo desde el cual es posible asumir la
-
br Discussion A familial platelet disorder with a propensity
2019-04-30
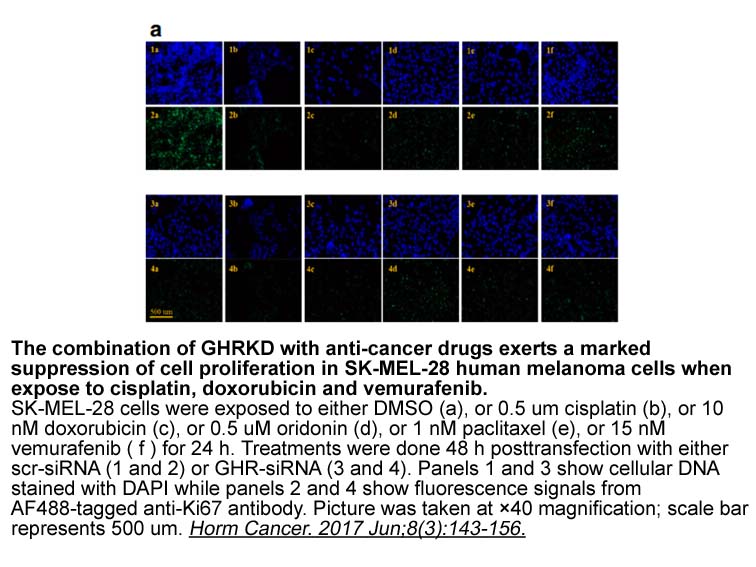
Discussion A familial platelet disorder with a propensity to myeloid malignancy (FPD/MM) was first reported in 1978 and since then approximately 30 pedigrees with RUNX1 germline mutations have been reported in the literature [9,11]. The RUNX1 gene is composed of 10 exons (1–6, 7A, 7B, 7C and 8).
-
When the analysis was limited to
2019-04-29
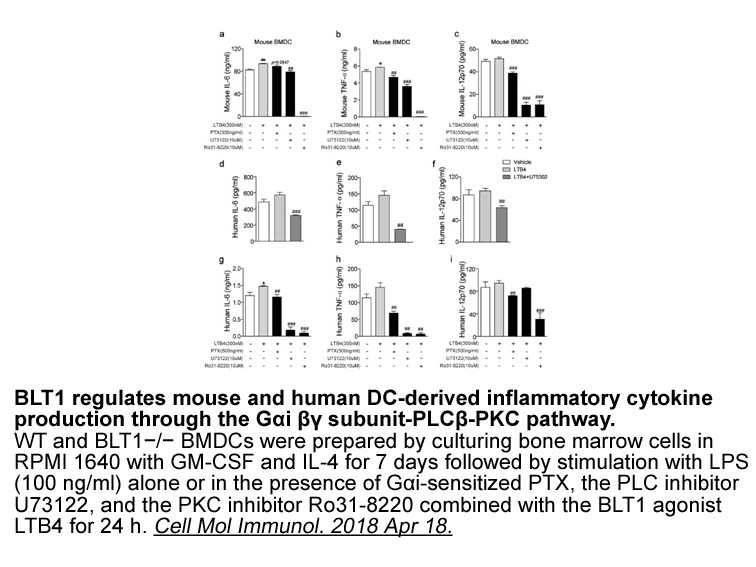
When the analysis was limited to subjects with a left ventricular ejection fraction ≤35% (Fig. 2), unvaccinated individuals received a significantly greater number of any ICD therapies during influenza season than vaccinated individuals (unadjusted IRR=5.8; P=0.045). However, significance was not re
-
hedgehog signaling br Gender difference in Brugada
2019-04-29
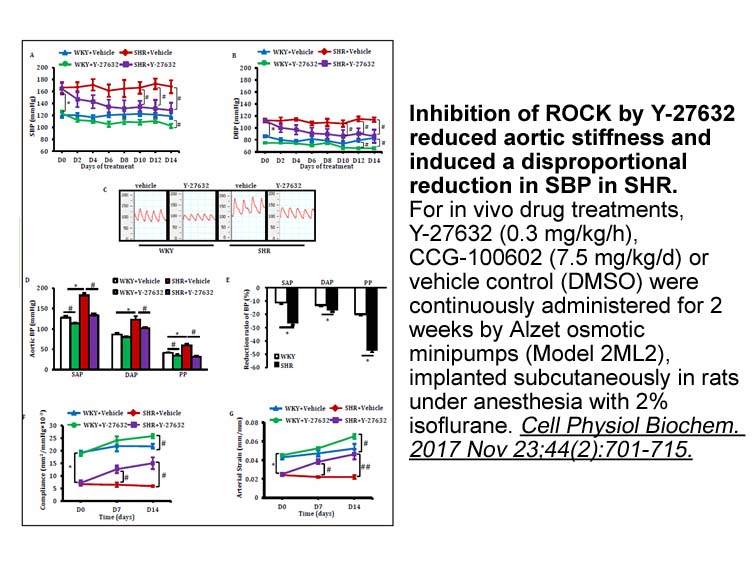
Gender difference in Brugada syndrome Despite the equal genetic transmission of the mutation between the sexes, the clinical phenotype is much more prevalent in men than in women. In Japan, community-based population studies have revealed that Brugada-pattern ECGs (coved or saddleback ST elevatio
-
The ablation instrument which was used in this study contain
2019-04-29
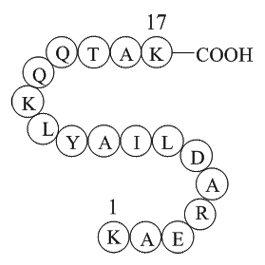
The ablation instrument which was used in this Phos-tag study contained a bipolar, articulated extendable electrode with multiple thermocouples and a proprietary generator (Fig. 1). The electrode provides a site specific and controllable ablation. The possibility to articulate and extend the electro
-
br Materials and methods br Results br Discussion To
2019-04-29
Materials and methods Results Discussion To our best knowledge, a multi-modality approach to systematically characterize an OS in vivo orthotopic model, using an aggressive OS cell line has not been previously reported. The advantage of using multi-modality approach i.e. BLI, histopathology
-
br Pathogenesis of the increased osteoclast
2019-04-29

Pathogenesis of the increased osteoclast activity in myeloma Histologic studies of bone biopsies from patients with MM demonstrate that increased OCL activity occurs adjacent to MM cells, suggesting that bone destruction in MM is a local event. This has led to the hypothesis that local cytokines
15864 records 1027/1058 page Previous Next First page 上5页 10261027102810291030 下5页 Last page